November 11, 2025
Why is the Gastric Cancer burden rising despite falling rates?
This November, during Gastric Cancer Awareness Month, we are highlighting a crucial public health paradox.

While the individual risk (incidence rate) of getting gastric cancer is decreasing globally, the absolute number of patients is rising due to population growth and ageing.
The Problem:
Fewer people per capita are getting the disease, yet the overall burden continues to grow. Over one million gastric cancer cases are estimated globally by 2040.
This rising caseload demands urgent action, especially in Europe, where many countries, particularly in Eastern Europe, still face high incidence rates but lack national screening programs.
There is a critical gap:
limited research evaluating the feasibility and effectiveness of gastric cancer screening in European populations.
The TOGAS project is bridging this gap. Our groundbreaking research across the EU is establishing evidence-based screening strategies.
With effective, tailored screening programs in place, we can finally stop the gastric cancer burden from rising.
October 8, 2025
International Conference in Riga on Personalized Cancer Screening Brings Together Leading European Experts
On October 8, the Great Hall of the University of Latvia hosted an international conference titled “Personalized Cancer Screening – Lessons Learned and Next Steps,” which brought together more than 100 leading researchers, clinicians, and policymakers from both Europe and beyond.

The conference was organized within the framework of the European Union-funded project “Joint Action on Implementation of Cancer Screening Programmes” (EUCanScreen). The aim of the EUCanScreen project is to promote cancer prevention and early detection, improve and standardize the effectiveness of cancer screening programmes in European countries, and strengthen the education and competencies of specialists.
The project focuses on improving existing breast, cervical, and colorectal cancer screening programmes, as well as laying the groundwork for the implementation of lung, prostate, and stomach cancer screening programmes. In order to promote the exchange of knowledge and good practice, the project has brought together experts from 29 European countries, including 25 EU Member States, as well as from Ukraine, Moldova, Norway, and Iceland.
The event featured five thematic sessions dedicated to different types of cancer and their screening approaches, the latest technologies, and the possibilities of using artificial intelligence in screening.
The conference was attended by experts from the Institute of Clinical and Preventive Medicine of the University of Latvia, Pauls Stradiņš Clinical University Hospital, Riga Stradiņš University, and other Latvian institutions, as well as from foreign countries, including Taiwan, who shared screening models and studies that are already being implemented outside Latvia, promoting international cooperation and exchange of experience.


The conference is an important step towards personalized cancer prevention, which will allow for improved early diagnosis, a reduction in the number of unnecessary examinations, and a more efficient use of healthcare resources across Europe.
October 6, 2025
Mārcis Leja presents GISTAR, TOGAS, EUROHELICAN, and EUCanScreen projects at UEG Week 2025
Professor Mārcis Leja, Director of the Institute of Clinical and Preventive Medicine at the Faculty of Medicine and Life Sciences of the University of Latvia, participated in the United European Gastroenterology Week (UEG Week) in Berlin on October 5. During the event, the International Healthy Stomach Initiative Group meeting was held, where Professor Leja presented the projects GISTAR, EUROHELICAN, TOGAS, and EUCanScreen.

In absolute terms, the total number of newly diagnosed cases of stomach cancer in Europe is increasing, and the Council of the European Union has recommended the introduction of screening programs in regions with high morbidity and mortality. Latvia corresponds fully to such unfavorable statistics. However, no organized stomach cancer screening programs have been established in Europe.
“Although there are several ways to reduce the burden of stomach cancer, for now it seems that the 'find and treat' strategy for Helicobacter pylori (H. pylori) infection may be the most appropriate for Europe. It is expected that projects such as GISTAR, EUROHELICAN, TOGAS, and EUCanScreen will provide the necessary evidence on which methods are best to reduce the risk of stomach cancer. The results will be important not only for Latvia but also for other European countries with similar statistics, as well as countries outside Europe. We already know that not only in Europe but also in the world, the GISTAR study data will provide significant new information,” emphasized Professor Mārcis Leja.
Stomach cancer is the fifth most common and fourth deadliest cancer in the world. In 2020, approximately one million new cases of stomach cancer were diagnosed, and almost 800,000 people died. The highest incidence rates of stomach cancer in the world after East Asia are in Central and Eastern Europe. In these regions, stomach cancer is most often detected late, so the probability of patients surviving the next five years is significantly worse—only 19%-30% of cases. Despite achievements in cancer therapy in recent years, five-year survival rates in most European countries have not improved significantly in recent decades.
About UEG Week: It was officially founded in 1992 when the first UEG Week was organized in Athens by the Hellenic Society of Gastroenterology under the leadership of Konstantinos Arvanitakas. In recent years, UEG Week has become one of the most prestigious events in medicine.
August 27, 2025
The GISTAR research center starts operations in Tukums
In August 2025, the GISTAR research center was opened in Tukums.
Those 647 residents who participated in the first phase of the study, which took place in 2016, will be invited for a follow-up examination.

With the aim of discovering and proving the effectiveness of new screening methods for the early detection and prevention of stomach and intestinal cancers in regions with high cancer prevalence, the GISTAR study (Gastric cancer prevention study by predicting atrophic gastritis) was initiated in October 2013. By the end of the inclusion period in 2023, 11,223 residents of Latvia had participated. This unique study in Europe, which takes place only in Latvia and is one of the longest and most comprehensive in Latvian medicine, is conducted by the Institute of Clinical and Preventive Medicine of the University of Latvia (LU KPMI) under the leadership of Professor and gastroenterologist Mārčs Leja. GISTAR study participants who were found to have the bacteria H. pylori in their stomach received a treatment course for its eradication. Currently, a follow-up survey of participants is being conducted, which will continue until February 2026. Invitations for follow-up visits have been completed in Cēsis (332 participants), Alūksne (331 participants), Ludza (546 participants), Saldus (548 participants), Rēzekne (1111 participants), and Jēkabpils (534 participants). In September, the survey of participants in Kuldīga is also planned to begin.
Some participants in the GISTAR study had received a treatment course for the eradication of H. pylori – now a specially designed breath test is being conducted at the research centers to determine whether the eradication of the bacteria has been successful, as H. pylori is considered one of the causes of stomach cancer. Blood tests have been taken from all participants, in which various inflammation and metabolism-related parameters are planned to be determined. These tests will also be performed on samples taken from participants nine years ago and stored frozen in the laboratory. The results of the old and new tests will be compared. It will be investigated whether there is any correlation between changes in the tests and health-related events with the use of H. pylori eradication medications.
All participants were offered to perform a fecal immunochemical test to determine the presence of hidden blood in the stool, and those with a positive test were advised to undergo a colonoscopy, or examination of the large intestine. During this examination, polyps can be detected, the removal of which reduces the risk of developing colorectal cancer.
Researcher, gastroenterologist Dr. med Danute Ražuka-Ebela from the Institute of Clinical and Preventive Medicine at the University of Latvia emphasized that there is currently a lack of effective stomach cancer screening methods in Europe, which makes the GISTAR study unique not only in Latvia but also on a European scale: “It seeks new strategies to reduce stomach cancer mortality in high-risk areas, including Latvia. Previous studies indicate that the eradication of the bacteria H. pylori, which resides in the stomach and promotes the development of stomach cancer, could reduce the number of deaths caused by stomach cancer by up to 40%. Therefore, it is crucial to find ways to implement this prevention in practice.”
"In absolute numbers, the total number of newly diagnosed stomach cancer cases in Europe is increasing, and the Council of the European Union has recommended implementing screening programs in regions with high incidence and mortality. Latvia fully aligns with such unfavorable statistics. However, there are still no organized stomach cancer screening programs established in Europe. Although there are several ways to reduce the burden of stomach cancer, it currently seems that the 'search and treat' strategy for Helicobacter (H.pylori) infection may be the most suitable for Europe. Currently, projects such as GISTAR, EUROHELICAN, TOGAS, and EUCanScreen are expected to provide the necessary evidence on which methods are best to reduce the risk of stomach cancer. The results obtained will be significant not only for Latvia but also for other European countries with similar statistics, as well as countries outside Europe. We already know that not only in Europe but also globally, the GISTAR study data will provide significant new information," emphasized Professor Mārcis Leja.
The data obtained in the GISTAR study are also used in other studies:
- TOGAS (Towards Gastric Screening implementation in the European Union);
- EUROHELICAN (Accelerating gastric cancer reduction in Europe through Helicobacter pylori eradication);
- EUCanScreen (European Joint Action on Cancer Screening).
Before the opening of the GISTAR center in Tukums, training was held for its employees, during which new employees were introduced to the research website, data usage and security regulations, the GISTAR data management system, the research protocol, the inclusion criteria for research participants, and practiced standard procedures.
The leading researcher of LU KPMI, Dr.sc.ing. Sergejs Paršutins, introduced the center's employees to data entry systems, security regulations, and data storage conditions, while LU KPMI senior expert Aiga Rūdule informed about the special project requirements, expected results, and quality control, as well as the design, goals, and objectives of the GISTAR study.
A lecture on the possibilities of reducing mortality caused by stomach cancer and the European perspective on these issues was delivered by the scientific leader of the GISTAR study, LU KPMI director, gastroenterologist, and professor at the University of Latvia, Mārcis Leja.


The project "Towards Gastric Cancer Screening Implementation in the European Union" (TOGAS) has received funding from the European Union program EU4Health under grant agreement No. 101101252.
The views and opinions expressed here reflect only the author's perspective and do not represent the position of the European Union or the European Health and Digital Executive Agency (HaDEA). The European Union and the funding body bear no responsibility for them.
July 17, 2025
Within the GISTAR study, 262 participants were surveyed in Dobele
The GISTAR study center in Dobele was first opened in 2016, and nine years later, the study center was opened for the second time, where 262 study participants arrived for the survey by June 30, 2025. The study results will be used in the TOGAS, EUROHELICAN, EUCanScreen projects and for the implementation of gastric cancer screening guidelines in the European Union.

With the aim of discovering and proving the effectiveness of new screening methods for the early detection and prevention of stomach and intestinal cancers in high cancer prevalence regions, the GISTAR study (Gastric cancer prevention study by predicting atrophic gastritis) was launched in Latvia in October 2013. By the end of the inclusion period in 2023, 11,223 residents of Latvia had participated. This unique study in Europe, which takes place only in Latvia and is one of the longest and most comprehensive in Latvian medicine, is conducted by the Institute of Clinical and Preventive Medicine of the University of Latvia (LU KPMI) under the leadership of Professor and gastroenterologist Mārčs Leja.
GISTAR study participants who were found to have the bacteria H. pylori in their stomach received a treatment course for its eradication. Currently, a follow-up survey of participants is being conducted, which will continue until February 2026. Invitations for follow-up visits have been completed in Cēsis (332 participants), Alūksne (331 participants), Ludza (546 participants), Saldus (548 participants), Rēzekne (1111 participants), and Jēkabpils (534 participants). In a few months, participant surveys will begin in Tukums and Kuldīga.
The data obtained in the GISTAR study are also used in other studies:
• TOGAS (Towards Gastric Screening implementation in the European Union);
• EUROHELICAN (Accelerating gastric cancer reduction in Europe through Helicobacter pylori eradication);
• EUCanScreen (European Joint Action on Cancer Screening).
Before the center opened in Dobele, training was held for its staff, during which new employees were introduced to the study's website, data usage and security regulations, the GISTAR data management system, the study protocol, the inclusion criteria for study participants, and practiced standard procedures.


The project "Towards Gastric Cancer Screening Implementation in the European Union" (TOGAS) has received funding from the European Union program EU4Health under grant agreement No. 101101252.
The views and opinions expressed here reflect only the author's perspective and do not represent the position of the European Union or the European Health and Digital Executive Agency (HaDEA). The European Union and the funding agency bear no responsibility for them.
July 1, 2025
In Jēkabpils, one phase of the GISTAR study has been successfully implemented, with 534 participants surveyed.
The GISTAR study center in Jēkabpils opened for the first time in 2019. Six years later, the study center opened again, where 534 participants arrived for the survey by June 30, 2025. The study results will be used in the TOGAS, EUROHELICAN, and EUCanScreen projects and for implementing gastric cancer screening guidelines in the European Union.

The GISTAR study (Gastric cancer prevention study by predicting atrophic gastritis/intestinal tumor prevention study, early detection of atrophic gastritis and colorectal damage) launched in October 2013 aims to discover and prove the effectiveness of new screening methods for early detection and prevention of gastric and intestinal cancers in regions with high cancer prevalence. In February 2025, the GISTAR study center opened in Jēkabpils. Residents who participated in the first phase of the study, which took place in 2019-2020, were invited for a follow-up examination.
By the end of the inclusion period in 2023, 11,223 residents of Latvia have participated. This unique study in Europe, which takes place only in Latvia and is one of the longest and most comprehensive in Latvian medicine, is conducted by the Institute of Clinical and Preventive Medicine of the University of Latvia (LU KPMI) under the leadership of Professor and gastroenterologist Mārčs Leja. Participants found to have the H. pylori bacteria in their stomach received a treatment course for its eradication. A follow-up survey of participants is currently being conducted, which will continue until February 2026. Invitations for follow-up visits have been completed in Cēsis (332 participants), Alūksne (331 participants), Ludza (546 participants), Saldus (548 participants), and Rēzekne (774 participants). Participant surveys are currently taking place in Dobele, and in a few months, surveys will begin in Tukums and Kuldīga.
The data obtained in the GISTAR study are also used in other studies:
• TOGAS (Towards Gastric Screening implementation in the European Union);
• EUROHELICAN (Accelerating gastric cancer reduction in Europe through Helicobacter pylori eradication);
• EUCanScreen (European Joint Action on Cancer Screening).
Before the center opened, training was conducted for its staff, during which new employees were introduced to the study's website, data usage and security regulations, the GISTAR data management system, the study protocol, and the participant inclusion procedure, and practiced standard procedures.


The project "Towards Gastric Cancer Screening Implementation in the European Union" (TOGAS) has received funding from the European Union program EU4Health under grant agreement No. 101101252. The views and opinions expressed here reflect only the author's view(s) and do not necessarily reflect the position of the European Union or the European Health and Digital Executive Agency (HaDEA). The European Union and the funding agency are not responsible for them.
March 25, 2025
Introducing European Cancer Initiatives at the Congress in South Korea
From March 20 to 22, the International Symposium on Helicobacter and Upper Gastrointestinal Diseases (HUG 2025) was held in Seoul, South Korea. During this event, Danute Ražuka-Ebela, a researcher from the Clinical and Preventive Medicine Institute (LU MDZF KPMI) at the University of Latvia, presented efforts to reduce gastric cancer in Europe.

In her presentation, Danute Ražuka-Ebela first introduced the audience to the situation regarding gastric cancer in Europe, highlighting that it remains one of the deadliest forms of cancer. As a result, the European Union has taken an active role in the fight against gastric cancer by implementing the Beating Cancer Plan, launched in 2021. This initiative aims to improve prevention, treatment, and care within member states, recognizing the importance of early diagnosis. Furthermore, in December 2022, the European Commission announced the need to develop and implement a gastric cancer prevention strategy.
In her presentation, Danute Ražuka-Ebela discussed studies focused on gastric cancer prevention and early diagnosis in Europe, all of which are also being implemented in Latvia through LU MDZF KPMI or the GASTRO CENTER. One of the longest-running studies in the history of Latvian medicine is GISTAR, initiated in 2013, which has involved over 10,000 participants. The study evaluates the effectiveness of H. pylori eradication and pepsinogen testing in reducing gastric cancer mortality. Meanwhile, the TOGAS project (Towards Gastric Cancer Screening Implementation in the European Union), which includes several pilot projects and the surveying of GISTAR participants, aims to develop recommendations for the establishment of gastric cancer prevention and screening strategies.
Another ambitious project, EUCanScreen, focuses on standardizing cancer screening protocols across Europe. It involves 29 countries, including EU member states, Ukraine, Moldova, Norway, and Iceland. This project is anticipated to significantly impact gastric cancer prevention in the long term.
Artificial Intelligence (AI) is playing an increasingly significant role in cancer diagnostics. In the AIDA project, involving LU KPMI, initial AI models have successfully analyzed histopathological images with remarkable accuracy—over 85%—in identifying cancer; future models will also be developed for detecting precancerous conditions during endoscopy. These technological innovations enhance diagnostic efficiency and accuracy, which could potentially save thousands of lives.
"Thanks to extensive and diverse research, from primary prevention to the integration of AI, the tremendous work invested by both Latvian and European colleagues, and our collective efforts to improve the situation, Europe is making significant strides in reducing the burden of gastric cancer. With initiatives like these aimed at developing evidence-based programs, we can expect improvements in disease prevention and early diagnosis, which can help millions of people across Europe," stated Danute Ražuka-Ebela.
March 17, 2025
GISTAR Study Center Begins Operations in Dobele
In March 2025, the GISTAR study center was opened in Dobele. A follow-up examination will be offered to the 529 residents who participated in the first phase of the study, which took place in 2016–2017. The GISTAR study (Gastric cancer prevention study by predicting atrophic gastritis), which began in October 2013, aims to identify and validate the effectiveness of new screening methods for the early detection and prevention of gastric and intestinal cancers in regions with high cancer prevalence.

By the end of the enrollment period in 2023, a total of 11,223 residents of Latvia had participated. This study, unique in Europe and conducted only in Latvia, is one of the longest and most comprehensive studies in Latvian medical history. It is led by Professor Mārcis Leja, a gastroenterologist at the University of Latvia’s Institute of Clinical and Preventive Medicine (LU KPMI).
GISTAR study participants who were found to be infected with the stomach-dwelling bacterium Helicobacter pylori (H. pylori) received treatment aimed at eradicating the bacteria. The follow-up examinations are currently underway and will continue until February 2026. Follow-up invitations have already been completed in Cēsis (332 participants), Alūksne (331), Ludza (546), Saldus (548), and Rēzekne (1,111).
Some participants who received treatment for H. pylori eradication are now undergoing a specially developed breath test to determine whether the eradication was successful. H. pylori is considered one of the causes of gastric cancer. Blood samples have also been collected from all participants to measure various inflammation and metabolic parameters. These tests will also be conducted on samples collected nine years ago and stored frozen in a laboratory. The old and new results will be compared to study potential correlations between test changes, health events, and the use of H. pylori eradication medication.
All participants were offered a fecal immunochemical test to detect hidden blood in the stool. Those with positive results were recommended to undergo a colonoscopy—a procedure that can detect and remove polyps, thereby reducing the risk of colorectal cancer.
Dr. Danute Ražuka-Ebela, a researcher and gastroenterologist at LU KPMI, emphasized that currently there is no effective gastric cancer screening method in Europe, making the GISTAR study unique not only in Latvia but also at the European level:
“With this study, we are exploring new strategies for reducing gastric cancer mortality in high-risk areas such as Latvia. Previous research indicates that eradication of H. pylori, which promotes the development of gastric cancer, could reduce gastric cancer-related deaths by up to 40%. It is therefore crucial to find practical ways to implement such prevention.”
"In absolute numbers, the total number of newly diagnosed gastric cancer cases in Europe is increasing. The Council of the European Union has recommended implementing screening programs in regions with high incidence and mortality rates. Latvia fits this adverse statistical profile. However, no organized gastric cancer screening programs have been established in Europe so far. While several methods exist to reduce the burden of gastric cancer, the 'search and treat' strategy for H. pylori infection currently seems the most suitable for Europe. It is expected that projects such as GISTAR, EUROHELICAN, TOGAS, and EUCanScreen will provide the necessary evidence to determine the most effective methods for reducing gastric cancer risk. The results will be significant not only for Latvia but also for other European countries with similar statistics and even for countries outside Europe. We already know that GISTAR study data will provide important new insights both in Europe and globally,” emphasized Professor Mārcis Leja.
Data collected in the GISTAR study are also used in other research projects:
- TOGAS (Towards Gastric Screening implementation in the European Union)
- EUROHELICAN (Accelerating gastric cancer reduction in Europe through *Helicobacter pylori* eradication)
- EUCanScreen (European Joint Action on Cancer Screening)
Before the center’s opening, staff underwent training where they were introduced to the study website, data usage and security protocols, the GISTAR data management system, study protocols, participant enrollment procedures, and practiced standard operating procedures.
LU KPMI lead researcher Dr. sc. ing. Sergejs Paršutins introduced staff to data entry systems, security protocols, and data storage conditions. Senior expert Aiga Rūdule provided information about the project’s specific requirements, expected outcomes, and quality control, as well as the GISTAR study design, goals, and tasks.
A lecture on the potential to reduce gastric cancer mortality and the European perspective on this issue was delivered by the scientific leader of the GISTAR study, LU KPMI director, gastroenterologist, and University of Latvia professor Mārcis Leja.
February 19, 2025
The GISTAR research center starts operations in Jēkabpils
In February 2025, the GISTAR research center was opened in Jēkabpils. Residents who participated in the first round of the study, which took place in 2019-2020, will be invited to re-examination. year.

In October 2013, the GISTAR study (Gastric Cancer Prevention Study by Predicting Atrophic Gastritis/Gastrointestinal Tumor Prevention Study by Early Detection of Atrophic Gastritis and Colorectal Lesions) was launched with the aim of discovering and proving the effectiveness of new screening methods for the early detection and prevention of gastric and colorectal cancers in regions with high cancer prevalence. By the end of the inclusion period in 2023, 11,223 Latvian residents had participated.
This unique study in Europe, conducted only in Latvia and one of the longest and most comprehensive studies in Latvian medicine to date, is led by Professor Mārcis Leja, a gastroenterologist, at the Clinical and Preventive Medicine Institute (LU KPMI) of the University of Latvia.
Participants in the GISTAR study who were found to have *Helicobacter pylori* bacteria in their stomachs received a treatment course for its eradication. Currently, a follow-up examination of the participants is underway, which will continue until February 2026. Invitations for follow-up visits have been completed in Cēsis (332 participants), Alūksne (331 participants), Ludza (546 participants), Saldus (548 participants), and Rēzekne (1,111 participants).
Some participants in the GISTAR study had received a treatment course for *H. pylori* eradication – now, a specially developed breath test is being conducted at the research centers to determine whether the eradication was successful, as *H. pylori* is considered one of the main causes of gastric cancer. Blood samples have been taken from all participants, and various inflammation- and metabolism-related parameters will be measured. These analyses will also be performed on samples that were collected from participants nine years ago and are stored in frozen form in the laboratory. The results of the old and new analyses will be compared. The study will investigate whether there is any correlation between changes in the analysis results and health-related events, specifically related to the use of *H. pylori* eradication medications.
All participants were offered a fecal immunochemical test to detect hidden blood in their stools, and those who tested positive were advised to undergo a colonoscopy (colorectal examination). During this procedure, polyps or growths can be detected, and their removal reduces the risk of colorectal cancer development.
The data obtained from the GISTAR study are also being used in other research projects:
• TOGAS (Towards Gastric Screening Implementation in the European Union)
• EUROHELICAN (Accelerating Gastric Cancer Reduction in Europe through *Helicobacter pylori* Eradication)
• EUCanScreen (European Joint Action on Cancer Screening)
Before the center's opening, training sessions were held for its staff, during which new employees were introduced to the study's website, data usage and security rules, the GISTAR data management system, the study protocol, participant inclusion procedures, and standard procedures.
Dr. Sergejs Paršutins, the leading researcher at LU KPMI, introduced the staff to data entry systems, security protocols, and data storage requirements, while Aiga Rūdule, the senior expert at LU KPMI, informed them about the special project requirements, achievable results, and quality control, as well as the design, goals, and tasks of the GISTAR study.
The lecture on opportunities to reduce gastric cancer-related mortality and the European perspective on these issues was delivered by Professor Mārcis Leja, the scientific director of the GISTAR study, the director of LU KPMI, a gastroenterologist, and a professor at the University of Latvia.
The project "Towards Gastric Cancer Screening Implementation in the European Union" (TOGAS) has received funding from the European Union program EU4Health under grant agreement No. 101101252. The views and opinions expressed here reflect only the author's view(s) and do not necessarily reflect the position of the European Union or the European Health and Digital Executive Agency (HaDEA). The European Union and the funding agency are not responsible for them.


February 11, 2025
One of the stages of the GISTAR study was successfully implemented in Rezekne, 1111 participants were surveyed
The GISTAR research center in Rēzekne was opened for the first time in 2017, seven years later the research center was opened for the second time, where 1111 research participants came to the survey until February 6, 2025.

Photo: Matīss Markovskis
In October 2013, the GISTAR study (Gastric Cancer Prevention Study by Predicting Atrophic Gastritis/Gastrointestinal Tumor Prevention Study through Early Detection of Atrophic Gastritis and Colorectal Lesions) was launched with the goal of discovering and proving the effectiveness of new screening methods for the early detection and prevention of gastric and colorectal cancers in regions with high cancer prevalence. By the end of the inclusion period in 2023, 11,223 Latvian residents had participated.
This unique study in Europe, which is conducted only in Latvia and is one of the longest and most comprehensive studies in Latvian medicine, is led by Professor Mārcis Leja, a gastroenterologist, at the Clinical and Preventive Medicine Institute (LU KPMI) of the University of Latvia.
Participants in the GISTAR study who were found to have *Helicobacter pylori* bacteria in their stomachs received a treatment course to eradicate the bacteria. Currently, a follow-up examination is being conducted for individuals aged 40 to 64, which will continue until February 2026. Follow-up invitations have been completed in Cēsis (332 participants), Alūksne (331 participants), Ludza (546 participants), and Saldus (548 participants).
Some participants in the GISTAR study had already received a treatment course for *H. pylori* eradication – now, a special breath test is being carried out at the research centers to determine if the eradication was successful. Blood samples have been taken from all participants, and in the near future, various inflammation and metabolism-related parameters will be analyzed. These analyses will also be performed on samples that were collected from participants nine years ago and are stored in the laboratory in frozen form. The results of the old and new analyses will be compared. It will be investigated whether there is any correlation between changes in the analysis results and health-related events with the use of *H. pylori* eradication medications. Additionally, the study will assess whether there is any correlation with cases where *H. pylori* infection was not treated.
All participants were offered a fecal immunochemical test to detect hidden blood in their stools, and those with a positive result were advised to undergo a colonoscopy (colon examination). During this procedure, polyps or growths can be detected, and their removal reduces the risk of developing colorectal cancer.
Dr. Danute Ražuka-Ebela, a researcher at the Clinical and Preventive Medicine Institute of the University of Latvia and a gastroenterologist, emphasized that there is currently no effective stomach cancer screening method in Europe, which makes the GISTAR study unique not only in Latvia but also in Europe: "With its help, new strategies are being sought to reduce gastric cancer mortality in high-risk areas, such as Latvia. Previous studies suggest that the eradication of *Helicobacter pylori*, which resides in the stomach and promotes the development of gastric cancer, could reduce gastric cancer-related deaths by as much as 40%. Therefore, it is essential to find ways to implement this prevention in practice."
"In absolute numbers, the total number of newly diagnosed gastric cancer cases in Europe is increasing, and the European Union Council has recommended implementing screening programs in regions with high incidence and mortality. Latvia fully fits such an adverse statistic. However, Europe still does not have any organized gastric cancer screening programs. While there are several ways to reduce the burden of gastric cancer, it currently seems that the 'search and treat' strategy for *Helicobacter pylori* infection could be the most appropriate for Europe. Currently, projects such as GISTAR, EUROHELICAN, TOGAS, and EUCanScreen are expected to provide the necessary evidence for the most suitable gastric cancer risk reduction method. The results obtained will be significant not only for Latvia but also for other European countries with similar statistics, as well as for countries outside of Europe. We already know that the data from the GISTAR study will provide essential new information not only for Europe but also for the world," emphasized Professor Mārcis Leja.
4th of February, 2025
LU KPMI continues research in the prevention of oncological diseases

February 4 is World Cancer Day – in this global initiative launched by the International Union Against Cancer, the Clinical and Preventive Medicine Institute (LU KPMI) of the University of Latvia also participates. Over its eight years of existence, LU KPMI has made a significant contribution to international and national scientific projects in the field of oncology.
One of these is the GISTAR project for stomach cancer prevention, the only one of its kind in Europe, whose data will largely serve as the foundation for developing stomach cancer prevention guidelines not only in Latvia but also in Europe.
This project, which has involved more than 11,000 participants from various regions of Latvia over 11 years, was initiated in cooperation with the International Agency for Research on Cancer and will continue until 2026.
In June 2024, LU KPMI began work on the large-scale EUCanScreen project. It is the largest European Commission joint action project to date, led by LU KPMI.
The project brings together 97 institutions from 29 countries and is one of the most significant European Commission initiatives in the field of cancer prevention. Its goal is to promote accessible, effective, and equitable screening across all European countries, including Latvia. One of the EUCanScreen project’s priorities is to increase public awareness of the importance of early diagnosis of breast, cervical, and colorectal cancers, as well as to develop recommendations for implementing screening for lung, stomach, and prostate cancers.
In March 2023, LU KPMI began implementing the TOGAS project. More than 20 partners from 14 European countries are collaborating on the introduction of stomach cancer prevention measures in all member states, developing unified guidelines for introducing stomach cancer screening in European countries and recommendations for evaluating its effectiveness. To achieve this, three large-scale pilot studies have been launched, which will help develop various methods for stomach cancer screening and early diagnosis. This is crucial because there is currently no effective stomach cancer screening method in Europe. Previous studies suggest that the eradication of the bacteria *Helicobacter Pylori*, which reside in the stomach and promote the development of stomach cancer, could reduce stomach cancer-related deaths by up to 40%, making it vital to find ways to implement this prevention in practice.
Emphasizing the need for timely cancer prevention, the director of LU KPMI, Professor Mārcis Leja, urges every Latvian citizen to pay special attention to their health and take advantage of the state's opportunities to undergo paid cancer screenings.
“Our country offers several screening programs that help diagnose the disease at an early stage. These are free of charge and available to anyone who meets certain criteria. Cancer is a serious disease, but early detection can be crucial. Together, we can reduce the impact of cancer on society. Take care of yourself and your loved ones – health is our greatest value!” emphasized Professor Mārcis Leja.

November 25, 2024
Marcis Leja presents research conducted in Europe on reducing cancer-related mortality in the USA.
From November 20-21, the National Cancer Center of the USA organized the annual CISNET (Cancer Intervention and Modelling Network) consortium forum in Rockville, where Professor Leja, a leading researcher at the Faculty of Medicine and Life Sciences, presented the achievements of the GISTAR, EUROHELICAN, and TOGAS projects.

CISNET is an international network of experts for intervention modeling aimed at reducing cancer-related mortality. This type of mathematical modeling allows for the identification of the most cost-effective strategies for planning measures in the fight against cancer.
Professor M. Leja was invited to present the results achieved in one of the working groups focusing on the modeling of gastric cancer (Gastric Cancer Modeling Group), addressing the GISTAR study conducted in Latvia and its results. In his virtual presentation, M. Leja discussed not only GISTAR but also ongoing related EU-funded projects, including EUROHELICAN and TOGAS, mentioning the plans intended to be implemented in the EUCanScreen project in the field of gastric cancer prevention.
"The presentation was highly appreciated, acknowledging that studies conducted in Europe have allowed for the acquisition of data that is currently not available in the USA. The issues mentioned in the presentation could mark the beginning of expanding existing collaboration with the National Cancer Institute of the USA and initiating partnerships with other US research organizations," emphasized M. Leja.
November 6, 2024
TOGAS Achieves Significant Progress in Gastric Cancer Prevention and Outreach Efforts
At this stage, TOGAS has made measurable progress and remains on track to achieve its planned outcomes, providing valuable insights into gastric cancer prevention. Public engagement and awareness initiatives have broadened knowledge through an online presence, educational materials, and targeted newsletters, reaching approximately 4,500 subscribers and producing 11 publications, including open-access formats.

Scientific contributions consist of findings presented at conferences and initial results published in peer-reviewed journals, enhancing Europe’s knowledge base on gastric cancer. Outreach efforts included a consortium meeting with 50 members, a conference with 84 in-person attendees and 11 online participants, and an outreach meeting featuring around 40 specialists, which facilitated feedback from stakeholders and EU representatives regarding project findings and applications.
Validated methodologies were achieved through WP3 (Evaluation), confirming the reliability of pilot studies, while early results from WP4 (Pilot studies) demonstrated adaptable screening methods suitable for diverse EU settings.
As we move forward, we have identified and are addressing risks, considering potential adjustments, and remain confident in meeting the project's planned objectives within the designated timeframe.
In conclusion, we would like to remind you that the achieved results pertain to the first 18-month period of the project and do not include the subsequent conference in September 2024.
November 1, 2024
November is Gastric Cancer Awareness Month
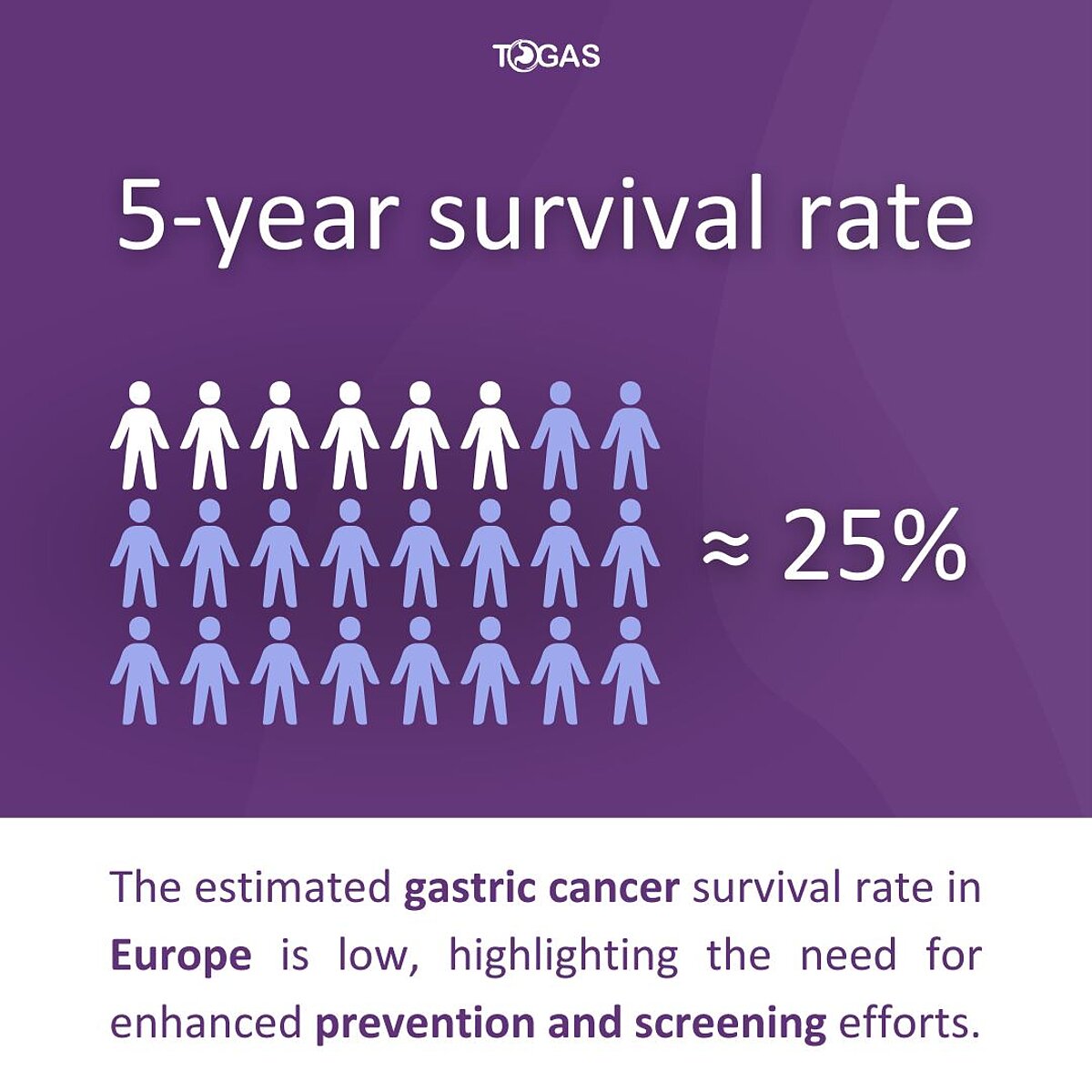
With a 5-year survival rate of just about 25% across Europe, gastric cancer remains a serious health concern. Despite rising case numbers, there is still no organized screening program in the EU. Early detection is crucial, as late-stage diagnoses contribute to the high mortality rate.
The TOGAS Project is working to change this by developing innovative screening and prevention strategies, including Helicobacter pylori (H. pylori) elimination. These efforts aim to reduce gastric cancer mortality by 40%.
Learn about the recent advances in clinical practice from the article by Prof. Mārcis Leja, leading the TOGAS research group:
https://gut.bmj.com/content/early/2024/09/04/gutjnl-2024-332705
And a big thank you to other projects, EUROHELICAN and EUCanScreen, for contributing to our shared objective.
October 4, 2024
Noticing an International Conference on Improving Cancer Screening
Last week of September marked a significant turning point in the EUCanScreen project aimed at enhancing cancer screening across Europe.

Photo: Toms Grīnbergs, UL
It was crucial for the European Joint Action on Cancer Screening (JA EUCanScreen) project, dedicated to strengthening cancer screening systems throughout Europe. The project's goal is to save lives by detecting cancer early. The event highlighted the project's main objective: to reduce the mortality rate from cancer by improving screening practices and promoting collaboration among European countries.
From September 24 to 27, 2024, more than 80 organizations and 200 participants from 28 European countries gathered at the University of Latvia's Science House in Riga to share insights and strategies. Project coordinators, led by Mārcis Leja from the University of Latvia's Faculty of Medicine and Life Sciences Clinical and Preventive Medicine Institute (referred to as LU KPMI), successfully organized the main sessions, ensuring a productive week in the fight against cancer.
Apostolos Vantarakis, one of the project's key leaders, emphasized, "Cancer is different in each country," pointing to the need to tailor strategies to regional contexts.
Mārcis Leja, director of LU KPMI, underscored Latvia's role: "Our biggest challenge is to successfully manage the project throughout its implementation period and achieve the project's goals on a European scale. However, with this project, we also want to make significant progress specifically in Latvia—bringing cancer control closer to the level of more developed countries by revitalizing existing breast, cervical, and colorectal cancer screening programs and preparing for the implementation of new, cost-effective, and high-quality lung, prostate, and stomach cancer screening programs, as well as developing the training center infrastructure established within the project."
Throughout the week, several significant discussions took place, including on:
● The role of innovation in cancer screening, highlighting new technological solutions such as artificial intelligence that improve early cancer detection.
● Cross-border collaboration, discussing how countries can share best practices to build stronger and more effective screening systems.
● Improving data collection and resource allocation to ensure more equitable access to cancer screening across Europe.
In a session led by one of the project leaders, Urška Ivanuš from Ljubljana (Slovenia), it was emphasized: "We will implement screening programs that will lower cancer mortality rates." Her statements align with the broader theme of the event, highlighting the necessity of systematically improving cancer screening to ensure better outcomes for citizens of various countries.
Iris Lansdorp-Vogelaar, a senior expert at ERASMUS MC and one of the project leaders, stressed the importance of screening frequency, noting: "The frequency of screening is closely related to the number of deaths from cancer. The more regularly screening is conducted, the greater the chances of saving lives."
Statistics show that timely cancer detection significantly increases survival rates, but equitable access to screening has yet to be implemented across Europe. One of EUCanScreen's goals, therefore, is to ensure more accurate data collection to improve resource allocation and expand access to screening.
As part of its mission, EUCanScreen representatives will continue to urge healthcare policymakers and representatives of medical organizations to support this essential work.

Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or HADEA. Neither the European Union nor the granting authority can be held responsible for them.
September 19, 2024
The Institute of Clinical and Preventive Medicine at the Faculty of Medicine and Life Sciences of the University of Latvia is leading an extensive cancer screening project called "EUCanScreen," involving 29 countries.

From September 24 to 27, 2024, the Institute of Clinical and Preventive Medicine at the Faculty of Medicine and Life Sciences of the University of Latvia (LU KPMI) will host the launch event for the "EUCanScreen" project. This project, which brings together 97 institutions from 29 countries, is one of the most significant initiatives of the European Commission in the field of cancer control. During the event at the LU House of Sciences, located at 3 Jelgavas Street, Riga, medical and healthcare professionals will discuss ways to improve the effectiveness of cancer screening and prevention across Europe.
In 2022, there were 2.7 million new cases of cancer diagnosed in EU countries, and Latvia is among the countries with the highest mortality rates. Thus, early cancer diagnosis is a critical challenge in Latvia. By enhancing knowledge about cancer screening and subsequently organizing and refining national cancer screening programs, public health can be significantly improved, and the number of cancer-related deaths can be reduced. Additionally, this presents an opportunity for Latvia to learn from other countries and adopt their experiences.
“The implementation of the EUCanScreen project will ensure significant progress in improving the effectiveness of cancer screening programs and harmonizing their execution among European countries. The project also provides us with a unique opportunity to strengthen collaboration between national and regional institutions on a European scale, enhancing our ability to combat cancer with a systematic and coordinated approach,” emphasizes Health Minister Hosams Abu Meri.
The goal of the "EUCanScreen" project is to promote accessible and effective screening in all European countries, including Latvia. One of the project’s priorities is to increase public awareness about the importance of early diagnosis. Another priority is to develop new screening methods for lung and stomach cancer. Therefore, in collaboration with leading health institutions in Latvia, such as the Disease Prevention and Control Center (SPKC) and Rīga Stradiņš University (RSU), current screening programs will be improved, and broader public engagement will be ensured.
The project will focus on ensuring that breast, cervical, and colorectal cancer screenings meet the quality criteria established in Europe across all EU member states, as well as on the gradual implementation of three new cancer screening programs (for stomach, prostate, and lung cancer), notes Institute Director Prof. Mārcis Leja.
The project will pay special attention to the early diagnosis of the four most common types of cancer: breast, cervical, colorectal, and prostate cancer.

The project "Implementation of cancer screening programmes (EUCanScreen)," LU registration No. ZD2024/21709, is funded by the European Union's EU4Health program, Grant Agreement No. 101162959.
September 10, 2024
Experts Commend the Work Accomplished in the GISTAR Study
On September 10, 2024, the GISTAR study's Data and Safety Monitoring Board met in Porto, Portugal, to discuss the progress made in the project so far. Representatives from the University of Latvia's Faculty of Medicine and Life Sciences, Institute of Clinical and Preventive Medicine, who are leading this unique study in Europe and globally, reported on the study's progress and compliance with data processing safety and privacy practices, as well as participant interactions.

Photo by Nick Karvounis on Unsplash
Researchers involved in the GISTAR study, including Professor Mārcis Leja, Inese Poļaka, Danute Ražuka-Ebela from the University of Latvia’s Faculty of Medicine and Life Sciences, Jin Young Park from the International Agency for Research on Cancer (World Health Organization), and GISTAR project coordinator Līga Krauze, also participated in the meeting.
GISTAR is a multicenter randomized study aimed at reducing mortality from gastric cancer through Helicobacter pylori (H. pylori) eradication and pepsinogen level determination. It seeks to discover new strategies to reduce gastric cancer mortality in high-risk areas. The study, which is conducted exclusively in Latvia, is led by the University of Latvia's Institute of Clinical and Preventive Medicine.
During the meeting, the GISTAR study's Data and Safety Monitoring Board praised the team's efforts to date, highlighting their successful focus on patient interests, safety, and privacy. The board also recommended the continuation of monitoring for enrolled participants as planned.
The GISTAR study is not only unique to Latvia but also to Europe, and it has gained international recognition. Some participants in the GISTAR study received a treatment course to eradicate H. pylori, and now breath tests are being conducted at study centers to determine the treatment’s success. Blood samples were taken from all participants, and various inflammation and metabolism-related parameters will be determined in the near future. These analyses will also be performed on samples collected five to eleven years ago, which are stored frozen in the laboratory. The old and new test results will be compared to investigate whether there is any connection between blood test changes and health events related to the use of H. pylori eradication medications. Additionally, the study will evaluate whether there are any correlations with untreated H. pylori infections.
Participant recruitment for the GISTAR study began in October 2013 and continued until August 2023, with a total of 11,223 participants enrolled. Follow-ups began in March 2023 and will continue until February 2026 as part of the European Union-supported EUROHELICAN and TOGAS projects.
Since spring 2023, study centers in Ludza, Alūksne, Cēsis, and Saldus have been operating, inviting GISTAR participants who were included in the study from 2013 to 2015 for follow-up assessments. Currently, the center in Rēzekne is still operating, and a new center opened in Madona on September 5. Over 2,000 participants have attended follow-up appointments so far. At present, extensive work is underway analyzing the data collected.
The members of the GISTAR study’s Data and Safety Monitoring Board are experts in medicine, data processing, and related fields:
- Chair Iris Lansdorp-Vogelaar, an Associate Professor at Erasmus University Rotterdam, is an expert in screening evaluation and modeling.
- Mangesh Thorat, a researcher at the University of London, specializes in cancer prevention.
- José C. Machado, a Professor at the University of Porto, is an expert in molecular pathology and immunology.
- Colm O'Morain, an Emeritus Professor at Trinity College Dublin, is an expert in clinical medicine.
- Jan Bornschein, from the University of Oxford, is an expert in gastroenterology.
- Fátima Carneiro, a pathology expert from the University of Porto.
- Manon Spaander, a Professor of Gastrointestinal Oncology at Erasmus University Rotterdam.
September 9, 2024
Where Are We with Stomach Cancer Screening in Europe in 2024?
The leading gastroenterology journal Gut* has published an article by Professor Mārcis Leja on the current status and future prospects of stomach cancer screening in Europe.

Although the Council of the European Union has recommended the introduction of stomach cancer screening, guidelines on how to implement this process still need to be developed. The "search and treat" strategy for *Helicobacter pylori* (H. pylori), which involves actively detecting the infection and eradicating it if the test is positive, is the most promising strategy for Europe. The article also provides an overview of current implementation studies in Europe: GISTAR, Eurohelican, TOGAS, EUCanScreen.
SUMMARY
The absolute number of stomach cancer cases in Europe is increasing every year. The Council of the European Union has recommended introducing stomach cancer screening in countries or regions with high incidence and mortality rates. However, as of 2024, no organized stomach cancer screening program has been launched in Europe.
There are several ways to reduce the burden of stomach cancer, but the *Helicobacter pylori* "search and treat" strategy seems the most suitable for Europe. It should be noted that this strategy would involve increased use of antibiotics.
In the European Union, only organized population-based cancer screening is recommended, so stomach cancer screening must meet the criteria of an organized screening program. Several aspects of screening organization must be considered before fully implementing stomach cancer prevention in Europe, including the target age group, types of tests, H. pylori eradication regimens, and monitoring strategies. Projects such as GISTAR, EUROHELICAN, TOGAS, and EUCanScreen are expected to provide the necessary evidence. Feedback from policymakers and potential target groups, including vulnerable populations, will be crucial for program planning.
This article provides an overview of recent decisions by European institutions, progress in the implementation of stomach cancer screening in Europe, and the challenges ahead. Finally, a potential algorithm for stomach cancer screening in Europe is proposed.
*Gut is a leading international journal in gastroenterology and hepatology, known for publishing high-quality clinical research on the digestive tract, liver, bile ducts, and pancreas. *Gut* offers up-to-date, authoritative, and clinically relevant content in all areas of gastroenterology and hepatology. Articles regularly describe new disease mechanisms and new diagnostic and treatment strategies that are likely to influence clinical practice in the near future, authored by leading experts in the field.
You can read the full article here: https://gut.bmj.com/content/early/2024/09/04/gutjnl-2024-332705.full
September 5, 2024
GISTAR Research Center Opened in Madona as Part of the TOGAS Project
During the training, new staff members were introduced to the study's website, data usage and security regulations, the GISTAR data management system, the study protocol, the participant inclusion process, and practical training in all standard procedures.


The GISTAR study is unique in Europe and has also gained international recognition. Currently, participant follow-ups are being conducted within the European Commission-supported TOGAS project.
A lecture on reducing mortality from gastric cancer and the European perspective on these issues was delivered via Microsoft Teams by the scientific lead of the GISTAR study, Professor Mārcis Leja, a gastroenterologist and director of the University of Latvia's Faculty of Medicine and Life Sciences, Institute of Clinical and Preventive Medicine (LU KPMI).
LU KPMI leading researcher Dr. sc. ing. Sergejs Paršutins introduced center staff to data entry systems, security regulations, and data storage conditions, while LU KPMI senior expert Aiga Rūdule provided information on the specific project requirements, expected results, quality control, as well as the design, goals, and objectives of the GISTAR study.
It is expected that the results obtained during the project will help experts develop guidelines, and policymakers integrate H. pylori testing and eradication strategies into healthcare priorities, thereby reducing the incidence of gastric cancer in Europe.
In Latvia, the project is implemented by the University of Latvia's Faculty of Medicine and Life Sciences, Institute of Clinical and Preventive Medicine (LU KPMI). Key partners include the National Institute of Public Health (NIJZ) in Slovenia, the International Agency for Research on Cancer of the World Health Organization (IARC/WHO) in France, the Nantes University Hospital (France), and the Dr. Adolf Drolc Community Healthcare Center in Maribor, Slovenia. The project will continue until the end of April 2025.
For more information about the TOGAS project - https://www.togas.lu.lv/
August 20, 2024
M. Leja Receives Recognition in South Africa for Support in Science
On August 16-17, 2024, the 1st International African Helicobacter and Microbiota Study Group (AHMSG) Inaugural Conference took place in Cape Town, South Africa. During the event, recognition was awarded to four scientists for their support, one of whom was Mārcis Leja, Director of the Institute of Clinical and Preventive Medicine at the University of Latvia.


This group was established two years ago in Nigeria, following the good experience of Europe, specifically modeled after the European Helicobacter and Microbiota Study Group. Two representatives from the European group, including one from Latvia, Professor Mārcis Leja, participated in its founding. Following the group's establishment, work began on developing the first African guidelines, with European representatives also participating in this effort.
The document was published earlier this year in the journal Digestive Diseases. This year, the conference saw broader representation from countries outside Africa, including scientists from Japan and the United States. The authors of the group's initiatives awarded their recognition to four guests for their support of the African countries' initiative, one of whom was M. Leja. The experience, including that of Latvia, which experts are ready to share with specialists on the African continent, was discussed. In his report, M. Leja provided an overview of the progress of the GISTAR, Eurohelican, TOGAS, and EUCanSCreen projects.
August 1, 2024
One of the stages of the GISTAR study led by the Institute of Clinical and Preventive Medicine of the University of Latvia was successfully implemented in Saldus, 548 participants were surveyed

The research center in Saldu was opened for the first time in 2015, nine years later the research center was opened for the second time, where 548 GISTAR participants came to the survey by the end of July 2024.
Various types of information were collected from the study participants about the events of the digestive system, heart and metabolic diseases since the first visit, lifestyle and eating habits and many other questions.
The GISTAR study is unique not only in Latvia, but also in Europe, and it has gained international attention. Some of the participants in the GISTAR study had received a course of treatment to eradicate H. pylori - now a special breath test was performed at the research centers to determine whether the eradication was successful. Blood tests were taken for all participants, in which various parameters related to inflammation and metabolism will be determined in the near future. These analyzes will also be performed on samples taken from participants nine years ago and stored frozen in the laboratory. The results of the old and new analyzes will be compared. Whether there is any relationship between changes in assays and health-related events with the use of H. pylori eradication medication will be investigated. It will also be evaluated whether there are any connections with the fact that the H. pylori infection is not treated.
All participants were offered a fecal immunochemical test to detect the presence of occult blood in the stool, and those who tested positive were advised to undergo a colonoscopy, or colon examination. During this examination, polyps or formations can be detected, the removal of which reduces the risk of developing colon cancer.
Researcher of the Institute of Clinical and Preventive Medicine of the University of Latvia, gastroenterologist Dr. med Danute Ražuka-Ebela says: "From the spring of 2023, the research centers in Ludza, Alūksne, Cēsis and Saldu have started and finished their operations, where GISTAR participants included in the study from 2014 and 2015 have been invited to the survey. Currently, the center in Rēzekne continues to operate. In total, more than 2,000 people came to the survey in the research centers (Ludzā 546, Alūksne 331, Cēsīs 332, Saldū 548, Rēzekne 340). Extensive work with the data and its analysis is currently underway. We are very grateful to the research participants for the time invested in the name of a common goal."
About GISTAR: A multicenter randomized trial to reduce gastric cancer mortality by H. pylori eradication and pepsinogen measurement. The study has been ongoing in Latvia since 2013.
The project "Accelerating the reduction of stomach cancer in Europe by eradicating H. pylori" (abbreviated as EUROHELICAN) is a project supported by the EU program "EU for Health" (EU4Health). As part of this, a population-based strategy for H. pylori testing and eradication is being evaluated. Infection with H. pylori significantly increases the risk of developing stomach cancer: most cases of stomach cancer are related to this infection. The project evaluates the use of the H. pylori testing and eradication strategy at the population level and various aspects of the strategy. The use of this strategy among young people is being studied in Slovenia. In Latvia, the long-term effects of this strategy are evaluated by surveying the participants of the GISTAR study.
In Latvia, the EUROHELICAN project is led by the Institute of Clinical and Preventive Medicine of the University of Latvia in cooperation with the National Institute of Public Health of Slovenia (The National Institute of Public Health - NIJZ), the International Agency for Research on Cancer of the World Health Organization (IARC) /WHO France), Nantes University Hospital (Nantes University Hospital, France) and Maribor Dr. Community Healthcare Center Dr. Adolf Drolc Maribor, Slovenia. The project will continue until the end of April 2025.

More about project EUROHELICAN: www.kpmi.lu.lv/petnieciba/petniecibas-projekti/eurohelican/
July 9, 2024
TOGAS research is being launched for the first time in Latvia, the first center has been opened in Rēzekne
The Institute of Clinical and Preventive Medicine of the University of Latvia, which is the leading partner of the study TOGAS - Towards Gastric Cancer screening in the European Union, launched the study "Detection and treatment of Helicobacter pylori infection in the population of young people" in Rēzekne.
Photo: Matīss Markovskis
The study will last until 01.04.2026. for the year. It is planned to include 300-400 participants aged 30-34.
"The goal of the TOGAS project is to develop recommendations for the introduction of gastric cancer screening in EU countries. In order to achieve this, three large-scale pilot studies are being conducted, with the help of which various aspects of gastric cancer screening and early diagnosis are being developed within 36 months," said Linda Mežmale, researcher at LU KPMI.
This type of research is needed because there is currently a lack of effective gastric cancer screening methods in Europe. Current research shows that the eradication of Helicobacter pylori, a bacteria that lives in the stomach and promotes the development of stomach cancer, could reduce the number of deaths caused by stomach cancer by as much as 40%, so it is important to find ways to implement this prevention in practice.
The pilot study - "Detection and treatment of Helicobacter pylori infection in the young population" is part of the international project "Towards Gastric Cancer Screening Implementation in the European Union, TOGAS", which aims to develop recommendations for gastric cancer screening for implementation in European Union (EU) countries. This pilot study will be implemented not only in Latvia, but also in other EU countries (Slovenia, Ireland, Croatia, Poland, Romania).
The aim of the study is to evaluate a population-based H. pylori test-and-treat strategy in a young population for gastric cancer prevention.
During the research, it is planned to detect H. pylori and eradicate the bacterium in a group of young people aged 30-34 years. Based on the obtained results, the participation level of the invited individuals in the study will be analyzed, the H. pylori eradication therapy and its side effects will be evaluated. The results of this study will provide essential information needed for the development and implementation of a gastric cancer screening program strategy in the European Union. Experts from the World Health Organization's International Agency for Research on Cancer will develop recommendations for the implementation of gastric cancer prevention measures and their effectiveness evaluation at the European level. The established guidelines and recommendations will help European countries to include these gastric cancer prevention measures in their health care priorities.

The project "Towards Gastric Cancer Screening Implementation in the European Union" (TOGAS) has received funding from the EU4Health program of the European Union in accordance with grant agreement no. 101101252.
The views and opinions expressed here reflect the views of the author(s) only and do not reflect the position of the European Union or the European Health and Digital Executive Agency (HaDEA). The European Union and the funding authority are not responsible for them.